शनिबार, वैशाख ६, २०८२
UK rolling out COVID-19 vaccine to public as world watches


DANICA KIRKA Associated Press (AP)
डिसेम्बर ८, २०२०
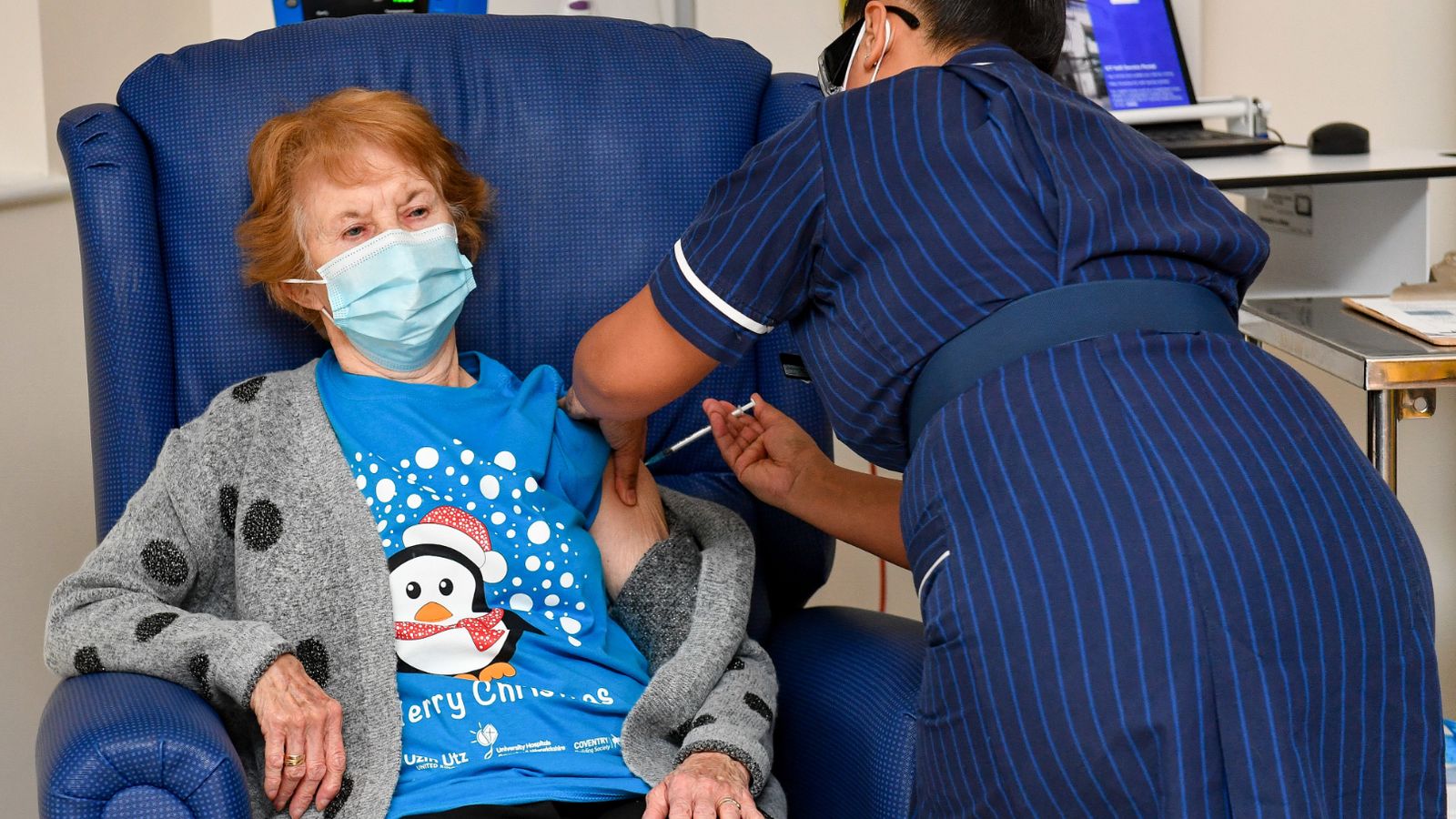
U.K. health authorities are rolling out the first doses of a widely tested and independently reviewed COVID-19 vaccine, starting a global immunization program that is expected to gain momentum as more serums win approval.
The first shot will come Tuesday at one of a network of hospital hubs around the country where the initial phase of the U.K. program will be rolled out on what has been dubbed “V-Day.” Public health officials are asking the public to be patient because only those who are most at risk from COVID-19 will be vaccinated in the early stages.
Medical staff will contact patients to arrange appointments, and most will have to wait until next year before there is enough vaccine to expand the program. “I think there’s every chance that we will look back on … (Tuesday) as marking a decisive turning point in the battle against coronavirus,” said Simon Stevens, the CEO of England’s National Health Service.
The first 800,000 doses are going to people over 80 who are either hospitalized or already have outpatient appointments scheduled, along with nursing home workers. Others will have to wait their turn. Among those older Britons scheduled to get vaccinated is Hari Shukla of Newcastle. “When I received the telephone call, I was very excited I got the opportunity of joining in and taking part in that,″ he said. “So we are very very pleased and happy and excited as well.″
Buckingham Palace refused to comment on reports that Queen Elizabeth II, 94, and her 99-year-old husband, Prince Philip, would be vaccinated as a public example of its safety. “Our goal is totally to protect every member of the population, Her Majesty, of course, as well,” Dr. June Raine, chief executive of Britain’s Medicines and Healthcare products Regulatory Agency, told the BBC.
Public health officials elsewhere are watching Britain’s rollout as they prepare for the unprecedented task of vaccinating billions of people to end a pandemic that has killed more than 1.5 million. While the U.K. has a well-developed infrastructure for delivering vaccines, it is geared to administer them to groups such as school children or pregnant women, not the whole population.
The U.K. is getting a head start on the project after British regulators on Dec. 2 gave emergency authorization to the vaccine produced by U.S. drugmaker Pfizer and Germany’s BioNTech. U.S. and European Union authorities are also reviewing the vaccine, alongside rival products developed by U.S. biotechnology company Moderna, and a collaboration between Oxford University and drugmaker AstraZeneca.
On Saturday, Russia began vaccinating thousands of doctors, teachers and others at dozens of centers in Moscow with its Sputnik V vaccine. That program is being viewed differently because Russia authorized use of Sputnik V last summer after it was tested in only a few dozen people. The first shipments of the Pfizer-BioNTech vaccine were delivered to a selected group of U.K. hospitals on Sunday.
At one of those facilities, Croydon University Hospital, south of London, staff members couldn’t so much as touch the vials, but they were thrilled to just have them in the building. “I’m so proud,” said Louise Coughlan, joint chief pharmacist at Croydon Health Services NHS Trust. The vaccine can’t arrive soon enough for the U.K., which has more than 61,000 COVID-19 related deaths – more than any other country has reported in Europe.
The U.K. has more than 1.7 million cases. The 800,000 doses are only a fraction of what is needed. The government is targeting more than 25 million people, or about 40% of the population, in the first phase of its vaccination program, which gives first priority to those who are highest risk from the disease.
After those over 80 and nursing home workers, the program will be expanded as the supply increases, with the vaccine offered roughly on the basis of age groups, starting with the oldest people. In England, the vaccine will be delivered at 50 hospital hubs in the first wave of the program, with more hospitals expected to offer it as the rollout ramps up. Northern Ireland, Scotland and Wales are making their own plans under the U.K.’s system of devolved administration.
Logistical issues are slowing the distribution of the Pfizer vaccine because it has to be stored at minus-70 degrees Celsius (minus-94 degrees Fahrenheit). The immunization program will be a “marathon not a sprint,” said professor Stephen Powis, medical director for NHS England.
Authorities also are focusing on large-scale distribution points because each package of vaccine contains 975 doses and they don’t want any to be wasted. The U.K. has agreed to buy millions of doses from seven different producers. Governments around the world are making agreements with multiple developers to ensure they lock in delivery of the products that are ultimately approved for widespread use.
सम्बन्धित सामग्रीहरू
हाम्रो सिफारिस

बेलायतको पृथक नेपाली संस्था जसको प्राथमिकता छ पुस्तान्तरण, च्यारिटी र वेलबिइङ (भिडियो)
मार्च १५, २०२५
ताजा अपडेट्स
ट्रेन्डिङ
- १
- २
- ३
- ४
- ५